This page is pretty heavy reading – just read if interested. Below is a link to the Dr Seyfried’s website, which he keeps up to date:
http://www.bc.edu/schools/cas/biology/facadmin/seyfried.html
Cancer as a metabolic disease: implications for novel therapeutics
2014 Thomas N Seyfried
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3941741/
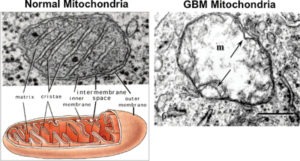
#1 hallmark of cancer is “broken” mitochondria –
NORMAL MITOCHONDRIA (left) These are in healthy cells.
- Normal mitochondria on left has elaborate cristae which are extensions of the inner membrane and contain what is needed for producing ATP (energy).
- Normal mitochondria produce energy by cellular respiration (also called oxidative phosphorylation, or OxPhos). This generates about 36 ATP per molecule of glucose metabolized, making it a much more efficient pathway than cancer cells use. This method requires oxygen, and it takes place inside mitochondria. Mitochondria can use glucose, fatty acids, amino acids & ketones.
CANCER CELLS MITCHONDRIA (right)
On the right is a mitochondria of a cancerous human glioblastoma – see the near total breakdown of cristae.
- Cancer cells have fewer and abnormal mitochondria –
- Cancer cells produce energy by glycolysis (also called fermentation) which is a relatively inefficient pathway that generates a net of just 2 ATP per molecule of glucose metabolized. (compare that with 36 ATP in a healthy cell) Fermentation does not require oxygen, and it takes place in a cell’s cytoplasm. Cancerous cells used fermentation so they can only use glucose & converts glucose to pyruvate.- and the pyruvate turns into lactate (lactic acid).
This is why a ketogenic diet fights cancer – cancer cells can only use glucose, but normal cells can use glucose or ketones. So, if you cut back on the blood glucose, you will still function well, but your cancer will starve.
Cancer can arise from any number of events that damage the cells’ mitochondria – virus’ or even many years of high insulin.
http://articles.mercola.com/sites/articles/archive/2013/06/16/ketogenic-diet-benefits.aspx
The following is a summary of the points in SEYFREIDs book- from this link:
http://www.diagnosisdiet.com/what-causes-cancer/
“Thomas Seyfried PhD, a brain cancer researcher with over 25 years of experience in the field, gave a groundbreaking presentation about cancer at the Ancestral Health Symposium held at Harvard Law School this past August. The three main take-home points of his talk:
1. Cancer is not caused by genetic mutations
2. Cancer is a mitochondrial disease
3. Cancer can be treated with ketogenic diets
DIFFERENCES BETWEEN HEALTHY CELLS AND CANCER CELLS
Cancer cells are very different from normal cells. Besides having fewer and defective mitochondria, they grow independently, ignoring the anti-growth signals and death cues that would normally keep healthy cells from getting out of control. Cancer cells create their own blood supply and can divide forever. Cancer cells lose many of the physical features of their mother cells; they are usually smaller, and may be disfigured or even shapeless. Sometimes they fuse with each other or with neighboring cells, creating strange hybrids. The most aggressive types of cancer cells invade local tissues and/or break loose and travel in the bloodstream to distant parts of the body (metastasize).
Healthy cells have stable DNA. DNA is the most important molecule in the body so it is well-protected. The DNA of healthy cells is not fragile. We would not have survived if it were. There are even “caretaker genes” that are designed to maintain and repair defects in DNA, because lots of things in the natural environment can injure DNA—even things we think of as healthy, such as sunlight.
Cancer cells have unstable DNA, which mutates easily and is therefore constantly changing. This is why there are so many mutations found in cancer cells.
Tumor cells are more vulnerable and fragile than healthy cells. This is actually how radiation and chemotherapy work. Radiation and chemotherapy are toxic to all cells, cancerous or not, but they are more toxic to tumor cells. If tumor cells were more robust than normal cells, these therapies would kill off all your healthy cells and only the big ugly tumor would survive.
Tumor cells are also more sensitive to heat (fever) and to starvation. When the body is stressed, the tumor cells are the first ones to go!!!
• A huge variety of things in the world—from viruses to radiation to chemicals to oxidation—can damage DNA and cause mutations. Seyfried quotes Nobel-prize winner Albert Szent-Györgyi:“…
“…it is getting more and more difficult to find something that is not carcinogenic.”
Here’s the thing: If you transplant mutated cancer cell DNA into a healthy cell, the healthy cell almost never becomes cancerous. Only 2 out of 24 experiments were successful in transforming normal cells into cancer cells by transplanted a cancer cell DNA into a healthy cell (and scientists couldn’t be sure that viral contamination wasn’t to blame). So, cancer is not truly a genetic disease.
Part 2 of 4 about Dr Seyfrieds book, Cancer as a Metabolic Disease.
The fascinating miniature world of the mitochondria–its role in our healthy cells and how mitochondria gone bad can lead to cancer.
Mitochondria turn the food we eat into energy. Mitochondria are beautifully complex structures living within almost all of our cells. Inside mitochondria are intricately folded membranes studded with special enzymes, fats, and proteins that are used to run elegant chemical reactions. These chemical reactions are what turn hamburgers into horsepower. Mitochondria float around in the outer region of the cell (called the cytoplasm).
Mitochondria are sophisticated power generators that break open the chemical bonds within food molecules to get at the energy inside. Chemical bonds consist of positive charges called protons and negative charges called electrons, which hold onto each other tightly. Mitochondria wrench the electrons away from the protons, and then funnel the electrons through an “electron transport chain”, creating current. This electrical energy is used to create ATP molecules, each of which includes a very high-energy phosphate bond. ATP (adenosine triphosphate) is like a miniature chemical battery; our cells can break ATP phosphate bonds apart whenever they need energy to do anything. Oxygen waits at the end of the ATP assembly line to catch the cascading electrons, and then binds to them, forming water as a harmless by-product. Because this process requires oxygen and results in a high energy phosphate bond, it is called “oxidative phosphorylation”, aka “respiration.”
Energy matters
The most important fundamental difference between normal cells and cancer cells is how they make energy.
Normal cells use the sophisticated process of respiration to efficiently turn any kind of nutrient (fat, carbohydrate, or protein) into high amounts of energy. This process requires oxygen and breaks food down completely into harmless carbon dioxide and water. Cancer cells use a primitive process called “fermentation” to inefficiently turn either glucose (primarily from carbohydrates) or the amino acid glutamine (from protein and also from proteins that are changed as food is processed) into small quantities of energy. [Note that fats cannot be fermented. This will be important later on.] This process does not require oxygen, and only partially breaks down food molecules into lactic acid and ammonia, which are toxic waste products.
Why would cancer cells do this, when there’s oxygen available?
Cancer cells are desperate. They can’t rely on their fancy respiration system because their mitochondria are damaged. Respiration cannot run smoothly unless the all of the delicate interior structures inside mitochondria are intact. Fermentation also takes place inside mitochondria, but the key difference is that fermentation is very simple and doesn’t require the complex inner machinery of the mitochondria- so fermentation provides cancer cells with energy even with cancer cells damaged mitochondria.
What kinds of things can damage our mitochondria?
• Radiation
• Cancer-causing chemicals
• Viruses
• Chronic inflammation
One way these things can cause problems for mitochondria is by generating reactive oxygen species (ROS), which damage respiration. You can think of ROS as unstable molecular pinballs, wreaking havoc with molecules around them, causing random damage wherever they strike.
It just so happens that some of the genes most strongly linked to cancer (“oncogenes”) are those that code for mitochondrial proteins. Some of the viruses most strongly linked to cancer are known to damage respiration:
• Kaposi’s sarcoma virus
• Human papilloma virus (cervical cancer)
• HIV
• Cytomegalovirus
- Epstein Barr
Cancer cells mitochondria
In what ways are cancer cell mitochondria damaged? Compared to healthy cells, cancer cells have:
• Fewer mitochondria per cell
• Misshapen mitochondria with unnaturally smooth inner surfaces
• Reduced activity of critical respiration enzymes such as cytochrome oxidase and ATPase.
• Smaller amounts of (deformed) cardiolipin– (a crucial mitochondrial fat)
• Less DNA within their mitochondria
• Leaky, uncoordinated electron transport chains that cause some precious energy to be wasted as heat instead of turned into ATP. [This abnormal situation is called “uncoupling.” It has been shown that faster-growing tumors are actually warmer because of this effect.]
Malignant cancer cells have been shown to have substantially lower respiration rates compared to normal cells. In one study of human metastatic rectal cancer, the cancerous cells had respiration rates 70% lower than the surrounding normal cells.
How do damaged mitochondria switch from respiration to fermentation?
Under normal circumstances, the DNA inside the nucleus calls the shots and sends orders out to the mitochondria in the cytoplasm. However, if a mitochondrion is damaged, and respiration is endangered, the mitochondrion sends an SOS message to the nucleus saying “we don’t have enough energy…we need to begin fermentation!” It essentially tells the nucleus to activate fermentation genes instead of respiration genes. You can think of fermentation as a clunky backup generator. It triggers the following events:
A variety of genes spring into action—genes that code for proteins required to run fermentation instead of respiration. These same genes are also called “oncogenes” (genes that are associated with increased cancer risk). It is likely that the reason why genes needed to run fermentation are also the same genes associated with cancer is that fermentation (and/or lack of respiration) increases cancer risk.
While these fermentation/oncogenes are revving up, their respiration counterparts are gearing down.
Genes like p53, APE-1 and SMC4. code for DNA repair proteins and are associated with respiration. These same genes are also called “tumor suppressor genes” (genes that prevent cancer).
Mitochondrial mayhem – a downward spiral into cancer.
Being in full throttle fermentation mode with respiration only limping along has the following effects:
• Reactive oxygen species (ROS) are generated, causing random damage.
• Iron-sulfur complexes are injured. These are needed in the electron transport chain.
• P-glycoprotein is activated, which pumps toxic drugs out of cells. This can make tumor cells resistant to most chemotherapy.
• The ability of mitochondria to initiate programmed cell suicide (apoptosis) fails. When something serious goes wrong within a cell, it is the mitochondrion’s job to make sure the damaged cell dies out – to protect the organism. This is how cancer cells with all kinds of strange mutations survive; fermentation allows weird cells to live on.
• Calcium leaks out of mitochondria and into the cytoplasm. Proper calcium flow is critical to normal cell division because the mitotic spindle, which is the structure that helps chromosomes separate properly, is calcium-dependent. Faulty spindles increase the risk of lopsided cell divisions—with one daughter cell getting too many chromosomes and the other daughter cell not getting enough.
Let’s look at some mitochondria transplant results :
• Fusing cancer mitochondria into normal cells and then injecting these hybrid cells into animals produces tumors in 97% of animals!
• Transplanting normal cytoplasm (mitochondria) into tumor cells reduces cancerous behavior.
•radiation damages mitochondria.
• What these results boil down to is this: Damaged mitochondria can turn healthy cells into cancerous cells and healthy mitochondria can reverse cancerous behavior in tumor cells. This tells us that cancer is not a genetic disease. Cancer is a mitochondrial disease.
The bottom line about mitochondria and cancer
Any number of environmental hazards can damage mitochondria—these are the same kinds of things we typically think of as causing cancer. It’s our mitochondria we have to worry about. Mitochondria take care of our cells and our DNA. Studies show that mitochondrial damage happens first, and then genetic instability follows.
What can we do to protect our mitochondria and prevent cancer? What if we already have cancer—what then? Can mitochondrial damage be reversed, or at least reduced?
Few, if any other diagnoses cause so much emotional distress, both for people with cancer and for their loved ones.
This is partly due to the potentially deadly nature of the condition, and partly due to the misery associated with most conventional cancer treatments—surgery, chemotherapy, and radiation. And if you are told you have an incomprehensibly complex genetic disease that even doctors don’t understand, you are placed in a position of powerlessness—you feel like a helpless victim.
Standard Dietary Recommendations
To add to the potential for despair, there is tremendous confusion around the simple question of what people with cancer are supposed to eat.
Cancer has a sweet tooth
Nearly all tumors depend heavily on glucose for survival, which is how PET scans are able to find many tumors hiding in normal tissues. PET scans follow radioactive glucose as it travels through the bloodstream. Radio-labeled glucose accumulates in tumor tissue more than in the normal tissues surrounding it, and lights up on the scan.
There is a strong connection between high blood sugar (hyperglycemia), diabetes, and cancer. It is well-documented that the growth of brain tumors is more accelerated and prognosis is worse in animals and humans with higher blood glucose levels. Hyperglycemia is directly linked with poor prognosis in humans and is connected to the rapid growth of most malignant cancers.
High blood glucose raises insulin levels, which stimulates cancer cells to take in and use more glucose—this makes it easier for cancer cells to nourish themselves. Insulin also turns up the activity of the fermentation pathway
High blood glucose also raises levels of another circulating hormone called IGF-I (Insulin-like Growth Factor I). IGF-I turns on a chemical pathway that drives tumor cell growth. This pathway sets the stage for cells to multiply, escape death (“apoptosis”), and recruit their own blood supply (“angiogenesis”). Angiogenesis is required for tumors to grow beyond 2 millimeters in size (2 mm is a little less than 1/10th of an inch).
To make matters worse, the genes for this growth pathway are also turned up by the fermentation process. More glucose = more fermentation AND more insulin AND more IGF-I = more tumor growth.
In short, cancer is a disease of growth, and insulin is the mother of all growth hormones.
Cancer’s Achilles Heel
Regardless of which type of cancer you have, the hallmark of all cancer cells is damaged mitochondria. According to Dr. Seyfried, cancer is not a collection of unrelated diseases that each need to be treated individually, cancer is one disease—a mitochondrial disease—and diseased mitochondria prefer glucose and glutamine for fuel. This is cancer’s Achilles’ Heel. Healthy cells with healthy mitochondria are flexible and can adapt to just about any fuel source, but not cancer cells. In fact, the majority of cells in our body function best when they burn fat for energy. Cancer cells are bad at burning fat, because fat burning requires respiration, which requires healthy mitochondria!!
How does dietary restriction work?
If food is restricted (both by avoiding sugar & high carb foods and by intermittent fasting) it will lower blood glucose, then insulin and IGF-1 levels will also be lower, quieting the tumor driving genes and pathways described above. This means that fermentation sputters, it becomes harder for tumors to recruit new blood vessels, and tumor growth slows.
Under low blood glucose conditions, insulin’s opposite hormone, glucagon, kicks in.
Glucagon stimulates fat burning, which raises ketones and fatty acids in the blood. (Ketones and fatty acids are just breakdown products of fats.) Ketone bodies and fatty acids cannot be fermented; therefore cancer cells cannot use them for fuel. However, most healthy cells prefer to use fatty acids and ketones for energy. Glucose restriction is good for healthy cells.
Glucagon also keeps your blood sugar from dropping too low by turning on a process in the liver called “gluconeogenesis” (making glucose from scratch). This is why we never need to eat any carbohydrates—we are always able to make all the glucose we need out of proteins and fats. The brain cannot burn fatty acids but it can burn ketones, and under low glucose conditions, the brain gradually shifts from burning mostly glucose to burning mostly ketones.
The brain may still require a small percentage of glucose to function at its best, but there is always enough glucose in the bloodstream because of glucagon, and most other organs will pass up glucose under these conditions in order to let the brain have first dibs.
Cancer cells and healthy cells both have a molecule on their surfaces called GLUT-1. This glucose transporter ushers glucose out of the bloodstream and into cells. Interestingly, under low glucose conditions, healthy cells will create more of these transporters and display them on their surfaces so as to optimize their ability to obtain glucose. Even more fascinating is that cancer cells, which are damaged, and therefore less flexible and adaptable, are not able to do this. In fact, when glucose levels are low, cancer cells are even weaker than usual; not only can they not raise their GLUT-1 levels, their GLUT-1 levels actually drop. This is one more way that glucose restriction impairs cancer cells. Even though there is always some glucose in the bloodstream because of gluconeogenesis, cancer cells are less able to access it than healthy cells because they are damaged.
The oxidation/inflammation connection
When ketones are burned for energy instead of glucose, fewer “reactive oxygen species” (ROS) are generated. These are wild free radicals that cause “oxidative damage”—a type of damage that has been associated with numerous chronic diseases. This means that shifting the body from being a carbohydrate-burning machine to becoming a fat-burning machine reduces oxidative damage, and therefore potentially reduces risk for numerous chronic diseases. Diets that raise blood levels of ketones are considered by neurologists to be “neuroprotective.” They protect brain cells from harm.
One reason why “ketogenic diets” (diets that force the body to burn ketones instead of glucose) are under consideration for the treatment of so many neurological diseases–from autism to Alzheimer’s, multiple sclerosis, epilepsy, Parkinson’s Disease– is that the transition from glucose burning to ketone burning is powerfully anti-inflammatory. Seyfried writes:
“There is no drug therapy that I am aware of that can target as many proinflammatory mechanisms in the microenvironment as can DER (dietary energy restriction). I think real progress in tumor management will be achieved once patients and the oncology community come to recognize this fact.”
In fact, Dr. Seyfried says that it is inflammation which damages mitochondria and respiration in the first place, and therefore inflammation may be the true cause of cancer.
How to starve cancer cells
Food restriction reduces the incidence of cancer in laboratory animals.
Most cancer cells grow best when they have access to a combination of glucose and the amino acid glutamine. There are some types of cancer cells which do just fine without any glucose as a food source, because they are especially good at burning glutamine. Dr. Seyfried argues that this is why BOTH glucose (from dietary carbohydrates) AND glutamine (from dietary protein) need to be restricted in order to best target cancer cells.
Dr. Seyfried recommends a specially-formulated low-calorie “ketogenic” diet consisting of 80% calories from fat, with the rest of the calories (20%) being made up of protein + carbohydrate. This diet forces your cells to burn fat for energy. It contains enough protein for your cells to function properly, but no more. Excess protein means excess amino acids, and glutamine is an amino acid. The ketogenic diet has vegetables to provide critical anticancer vitamins & phytochemicals, but, according to Seyfried, calories must be kept low.- blood glucose levels respond to calorie intake as well as to carbohydrate intake.
The goal of this diet is to shift your body from burning mostly glucose (sugar) to burning mostly ketones (fat). Fat molecules get broken down into 3 fatty acid chains plus one molecule of glycerol. The fatty acids can be turned into ketones, and the glycerol backbone can be turned into glucose. [This is why even eating too much fat can raise blood sugar a little bit in some people. Carbohydrates are best at causing high blood sugar. Proteins can raise blood sugar (although not as easily and not as steeply) because some amino acids can be turned into glucose. Dietary fat is least likely to raise blood sugar, but it is not impossible, especially if you are eating more calories than you need.] The idea behind ketogenic diets is to restrict carbohydrate and protein so much that fat from the diet is broken down into ketones, which are burned by healthy cells for energy.
Summary of Dr. Seyfried’s recommendations for cancer patients
People following strict ketogenic diets to manage cancer need to monitor their blood sugar and blood ketones daily.
Dr. Seyfried recommends that blood sugar levels be allowed to fall into the 55-65 mg/dL range, and that ketones rise to 4.0 mM. He refers to this combination of values as “the zone of metabolic management.”
The quickest way to get into the therapeutic zone is by fasting (water only) for 3-5 days. During the induction phase, (harmless) carbohydrate withdrawal symptoms may occur, which typically include lightheadedness, nausea, and headaches.
He offers an alternative to this fasting induction: limit carbohydrates to less than 12 grams per day and limit protein to 0.8 to 1.2 grams per kg body weight per day (0.4 to 0.6 grams per pound body weight). With this less extreme plan, he says it may take up to several weeks to reach the recommended therapeutic zone values.
Once you are in the zone, he recommends you use your daily test results to fine-tune your caloric intake–i.e. see how many calories you can get away with while staying in the zone. Everyone’s metabolism is different, so some people can get away with more calories than others without falling out of the zone. He also recommends supplementing your diet with a multivitamin, calcium, omega-3’s and vitamin D.
If your cancer would benefit from surgical debulking, he recommends waiting until you have been on the ketogenic diet for at least a few weeks before undergoing surgery, if you can afford to wait. This is because the diet can reduce blood vessel mass, inflammation, and tumor size, making it easier for the surgeon to remove the tumor more cleanly.
Dr. Seyfried points out that vigorous exercise can raise blood sugar levels, and therefore he advises patients to “walk, not run.” Strenuous muscle activity releases lactic acid into the blood, which can be converted into glucose by the liver and released back into the bloodstream.
NOTE: Dr. Seyfried writes: “We do not believe that KD-R (restricted ketogenic dieting) alone will provide complete disease resolution for most patients.” He then goes on to discuss other strategies that can be combined with dietary restriction to optimize results–these will be covered in article 4.
Some Basic Precautions
All of your medications must be closely monitored by your physician because this diet can significantly affect required dosages.
This diet will not work if you are taking steroid medications such as dexamethasone (Decadron), because steroid medications raise blood sugar. It may also not work if you are receiving intravenous medications which contain glucose.
It is very important to have the support of your household and your physician if you embark on such a plan, because it requires close monitoring, discipline, and social support (my husband eats everything I make for all meals, but adds carbs – I have a taco salad, he has a taco, I have lettuce wrap, he has sandwich etc).
• DER (dietary energy restriction) triggers cancer cell death via apoptosis (programmed cell suicide), which is a natural, noninflammatory process that happens from within the cell, causing no collateral damage. Tumor cells that are being fed glucose/glutamine are resistant to apoptosis, but under ketogenic conditions, they become better able to undergo apoptosis again.
Ketogenic Diet and Its Role in Cancer Treatment
(Summary of Interview with Dr. Thomas Seyfried) By Dr. Joseph Mercola (DM: Dr. Joseph Mercola TS: Dr. Thomas Seyfried)
Introduction: DM: Welcome, everyone. This is Dr. Mercola, and today I am joined by Dr. Thomas Seyfried, who has been teaching neurogenetics and neurochemistry as it relates to cancer treatment at Yale University and Boston College for the past 25 years. He has written over 150 peer-reviewed scientific articles and book chapters, and has also published a book, Cancer as a Metabolic Disease: On the Origin, Management, and Prevention of Cancer. We are beyond delighted to have an expert such as yourself who’s really a leader and a pioneer in using diet as a way to address what is one of the most common diseases that’s now being faced by men and actually one of the most common causes of death.
TS: Thank you so much.
DM: … what motivated you to start doing research in this area.
TS: Well, we’ve been doing work research in epilepsy for many decades while I was at Yale and carried it through here in our work at Boston College. It turned out that the ketogenic diet had been used for quite some time for managing seizures in children. We were doing a lot of work on brain cancer in mice and epilepsy in mice. One of my students just thought it would be good to see whether or not ketogenic diets might also be effective against tumors, because they were targeting similar energy pathways. That was back in the late ‘90s. And then it became clear that the mechanisms and the processes were closely paralleled to what Otto Warburg had indicated in the 1920s.
DM: Has the clinical medicine accepted the ketogenic diet as the standard of care or at least a valuable option to the treatment of epilepsy at this point?
TS: Yeah. This is an interesting point… Jim Abrahams, started the Charlie Foundation for his son Charlie, who went through a near-death experience from seizures and was rescued using ketogenic diets. His colleague, Meryl Streep, the famous movie actress, became very involved in this. Now the ketogenic diet is receiving considerable attention in the epilepsy community as a first line of approach. Although this is still not widely accepted, the ketogenic diet is now recognized as an important component for the management of refractory seizures in children. The ketogenic diet is moving forward consistently in the field of epilepsy as an non-toxic, effective therapy for refractory seizures.
TS: It turns out that it is the glucose reduction that’s killing the cancer cells, and the normal cells are now transitioning over to ketones, which protect them from, if any, issues of hypoglycemia. DM: So, you’ve developed this process called metabolic control analysis. I’m wondering if you could describe what that is and how that relates to the treatment and prevention of cancer.
TS: Well, It’s basically a mathematical analysis of very complex integrated network systems… when we transition the body from one major fuel source to another major fuel source, there are major flux changes in the homeostasis of these pathways. That then links the approach to the concepts of metabolic control analysis, which essentially is the analysis of flux through different pathways to maintain global energy homeostasis.
DM: Many people believe or are under the impression that cancer’s primarily a genetic disease. We’ve sequenced the human genome, and there are these screens for that. It presents a lot of fear in many people, because they’ve inherited the cancer genes.
TS: Let me start off by addressing the issue of whether cancer is a genetic disease or whether it’s a mitochondrial metabolic disease. You know, one of the key issues here is that if you transplant the nucleus of a cancer cell into a normal cell, you don’t get cancer cells. You can actually get normal tissues and sometimes a whole normal organism from the nucleus of a cancer cell. Now, if the tumors are being driven by driver genes – all these kinds of mutations and things that we hear about – how is it possible that all of this is changed when you place this cancer nucleus into the cytoplasm of a cell with normal mitochondria? The gene theory cannot address this. Actually, a very few people inherit genes that predispose them to cancer. Most people inherit genes that prevent cancer. And those few genes that are inherited – the germ line like the BRCA1 mutations, B53, and a few other very rare cancers – these inherited mutations appear to disrupt the function of the mitochondria. The mitochondria are actually the central point in the origin of the disease. The mitochondria can be damaged not only by inherited mutations, thereby increasing the risk for someone to develop a particular type of cancer. But what we have is this impact through just living in our environment that damages mitochondria in various tissues. And it’s this irritation and damage to the tissue structure that leads to the alteration of function in the mitochondria of those cells, which then go on over a protracted period of time to develop cancers. So, it’s ultimately a disease of the mitochondrial energy metabolism, which is the origin of the disease. I’ve shown in my book and others have shown that once the mitochondria become dysfunctional or insufficient in ability, mutations will occur. These mutations are essentially downstream epiphenomenon of the destabilization of energy metabolism. This, in my mind, is one of the reasons why focusing on genomes of the disease will likely bear little, if any, fruit. The drugs that have been developed based on the genome projects have been largely ineffective in providing long-term care and are associated with toxic effects.
DM: Would it be fair to summarize that in the large portion of your work, you’ve identified that the primary tool for most cancers is sugar, and that if you restrict their fuel and provide alternate fuels for the person, you can dramatically reduce the rate of growth of the cancer? Is that a fair summary?
TS: Yeah, I think that’s a good summary. The problem is how to do that, how to implement a particular therapy that would lower blood glucose level. We know that there are certain risk factors that will increase our risk for cancer. There are certain factors in the environment that will increase our risk, and by simply avoiding those we can reduce our risk. But as you know, many people deal with the disease after they’ve had the disease. They’re not really worried about the risk factors until they get the disease. And then it becomes an issue of management. When we’re dealing with glucose and management, we know from a large number of studies that if respiration of the tumor is ineffective, the cells to survive must use an alternative source of energy, which is fermentation. We know that glucose is the primary fuel for fermentation. Fermentation becomes a primary energy-generating process in the tumor cell. By targeting the fuel for that process, we then have the capability of potentially managing the disease. Although that’s easy to say when you look at the overall picture, actually doing this can represent a number of challenges, such as how to effectively lower blood glucose levels without harming normal tissues in the body. And this is the problem for many cancer therapies. Drugs or whatever you do, you kill the cancer cell, but at the same time, you’re harming normal cells of the body. The strategy that we’ve taken is the use of these low-carb, high-fat diets… If you lower the blood sugar, (this is an easily measurable parameter. You can use a diabetic blood glucose meter.) And then you elevate these ketone bodies, which we as a species have evolved to burn ketones in the absence of food. We have within our bodies the capability of surviving long-term from the energy stores within our body. Those energy stores come from fat (which is metabolized to ketones), which is then an effective fuel as an alternative to glucose. It’s a fat breakdown product that can replace glucose as a major fuel for many of the organs and especially our brain. The tumor cells cannot use these ketone bodies because of their respiratory insufficiency. This represents an elegant, non-toxic way to target and marginalize tumor cells. It also allows us to lower glucose levels to quite low levels, because the ketones will protect the body against any hypoglycemia that might be induced by the therapies. All of the newer cells in the body will be transitioned to these ketones, thereby preventing them from damage from hypoglycemia. At the same time, the tumor cells are now marginalized and under tremendous metabolic stress. You need to bring the whole body into this metabolic state. This is not to say that certain drugs are not to be used. Because we know from our work that if you can get the body into this state, certain drugs – which would have been marginally effective when the body would have high glucose and the tumor cells are at their peak – now can be used in very low levels and be highly successful in targeting some of the more resistant tumor cells. It’s natural. It’s a very interesting kind of strategy. If it’s done right and implemented right, it has powerful therapeutic benefits on the majority of people who suffer from various kinds of cancers. Because all cancers have primarily the same metabolic defect.
DM: Yes. interestingly, they are similar with respect to the similar metabolic pathways that can be utilized to treat them. Let’s get into some of the specifics of the ketogenic diets, because I’m sure many people will be interested. It would also be fair to summarize that for the most part, the body has only two fuels: sugar and fat. The strategy that you’re doing is to lower the sugar that’s being consumed and being utilized and then shifting them over to the fats, so these ketone bodies are generated and permanently used. the challenge is to make this transition over- to upregulate the enzymes that burn the fat rather than burn the sugars?
TS: Yeah. Well, we have fat, carbohydrate, and protein. Of course, the proteins can be metabolized to glucose in the liver as well. So, proteins can be an energy source. But fats spare protein, which is nice. The body doesn’t start metabolizing protein for energy unless fat is pretty well depleted. yeah, as we transition, fats provide – fat is basically triglycerides – our storage fuel- this is a fuel that is now called on to provide energy when glucose becomes restricted. How long it takes to burn the stored glucose in our bodies actually could depend on the individual. There’s a lot of individual variation in this. Because you’re right: our blood sugar is replenished by each meal. Insulin drives the glucose into the tissues. If the tissues have excess glucose, this will be converted to glycogen and stored in our liver. If we stop eating, how long does it take to deplete our body of a ready source of carbs? You haven’t eaten for several days. What’s your blood sugar now? Because your glycogen reserve should be depleted. And yet we’re still seeing relatively high glucose like in 85 milligram per deciliter to something along these lines. And you say, “Wow, geez, I haven’t eaten anything for three days. How can this be?” I’ve done it on myself and on my students. It turns out how effective gluconeogenesis is. Gluconeogenesis can actually be generating glucose from other sources of proteins. Also, when people stop eating – we’re learning this now a little bit more from cancer patients basically – there’s a stress. It’s overall body stress. Corticoids, our glucocorticoids, can be generating glucose from metabolites in the body to maintain a steady level of glucose. So, there are a lot of factors – hormonal, emotional, and metabolic – that go into these kinds of transitions. We’re learning more and more that each individual has their own metabolic homeostatic state. They need to know for themselves by measuring their blood sugars and ketones what point are they in a new state of metabolic homeostasis, a state where ketones have reached the steady state level in the blood and glucose has reached a steady state lower level in the blood from before. Whether it’s therapeutic fasting (which is water-only dieting), it is a ketogenic diet, or any of these kinds of dietary manipulations, you want to know when your body has achieved a new metabolic state. Basically, we use ketones and glucose as the simplest measures of this new state. We also take blood. We look at lipids. We look at hormones and things like these. But the average person cannot do this.
DM: What parameters are you looking for with respect to your glucose and ketone levels, knowing that you’ve achieved the ketogenic state?
TS: Well, usually when we see the millimolar levels of ketones are equal to or higher than the millimolar levels of glucose in the blood. in a fasted or therapeutic state, this ratio is actually reversed. Ketones can actually become higher than glucose. Now, most normal people cannot elevate ketones much higher than six or seven millimolar in the blood. They will simply eliminate it. Urine will eliminate the ketones. (People will never reach ketoacidosis. This is a pathological state that’s seen in people with type 1 diabetes) What they can do is they can get their blood sugars down to two and a half millimolar or three, and then their ketones to up to three or four millimolar, where the ratio is now reversed. It’s this state that now brings the body into this new physiology.
DM: Can you translate those numbers to ones that we’re more familiar with, which is in milligrams per deciliter?
TS: Glucose will probably be about, say, 55 to 70 milligrams per deciliter. And we know we can achieve that. Just simply if you stop eating long enough, you can get your body into one of these states. There will be some fluctuations. But the fluctuations are all within a fairly narrow range. You can shoot up to 90 or 100 milligrams per deciliter occasionally. But basically during the course of the day, the stability of the glucose in and around 55 to, say, 70, you know. It’s not written in stone. But it certainly gives a guideline to the stability. We – my colleagues who do the epilepsy studies and myself – found that seizures can be managed quite effectively as long as you can keep the blood glucose low, steady, and stable. This is also the same for cancer. If you can keep the blood sugar low, stable, and steady, the tumor cells now (which are dependent on this glucose for their survival) are put under extreme metabolic stress. It’s how long you can stay in this state that will just determine how long you can put the pressure. And I find it sometimes surprising that those individuals with type 2 diabetes or even type 1 diabetes who do regular blood sugar monitoring with finger pricks and blood monitoring, this is part of their daily life. They do this because they know that if they don’t, they risk unconsciousness and various other problems. But for some reason, the cancer patients have more difficulty knowing that this is precisely what they have to do. Cancer patients can keep records just like diabetics do. When the child or the patient with epilepsy gets off the ketogenic diet or changes it, there’s a breakthrough seizure. Your lack of consistency is immediately seen and known. It’s unambiguous; a seizure is very recognizable. Whereas a cancer patient doesn’t see the immediate consequences of falling off of this steady state. That’s why we try to get them to measure it every day like a person with diabetes. DM: Do you recommend that cancer patients do this regular monitoring of their blood sugar? How many times a day do you advise it, and what are some of the goals that you’re looking for? Somewhere between the 55 and 70 range, as you said?
TS: Yeah, 55, 65, to 70, as long as it’s low and steady. Ketones are generally in the two to five millimolar range. You also have to recognize that if you become that level in ketones, a fluid elimination becomes a more prominent issue. Therefore, salt balances need to be required. This is why these diets should be done with careful monitoring by physicians or qualified health professionals. What I do simply is provide the guidelines that should be followed. But everybody is individual. They have to have a complete physical examination. They have to be told what to be aware of. I work with nutritionists and physicians. The problem with cancer patients is that many of the practitioners are unfamiliar with this whole approach, so there’s this tremendous gap. We have patients willing to do it. But we lack professionals that are trained or even understand the concepts of how to implement these kinds of approaches.
DM: Where are the guidelines that you’ve compiled? Are they available?
TS: Yeah, they’re in my book. And we’ve published a couple of papers that outline these guidelines and treatment strategies – oncology… I myself and some of my nutritionist and physician colleagues have co-published articles that give patients certain guidelines. They’re in my book. It tells you how to do this as best as we can. We’re learning ourselves more and more how individuals vary in their response to these treatments and how – some of my colleagues from Europe are realizing that – not everyone can make this transition readily. Some people require a fine tuning to the therapy, a gradual transition from one metabolic state to the other. Some people can jump in with both feet, going from a high-carb basic diet into a high-fat diet without any physiological ramifications. Others have tremendous difficulty doing this. They complain of heart-pounding or various kinds of physiological effects, which we know are consequences of just the shift from one metabolic state to the other. This is why it’s simple in concept but much more difficult in implementation.
if one is to use this therapy with medications, one needs to know what are the adverse effects that might happen if one will apply a medication at a particular dosage with the metabolic therapy as a concurrent treatment strategy.Clearly, we have a learning curve. We know that this will work. If it’s done correctly, we can see a rather dramatic effect. The problem is getting those dramatic and therapeutic effects in a more general way and a more predictable way to the majority of people who would like to use the approach. DM: One of the strategies we’ve been advocating for people to get healthier and optimize their body weight is something I’m sure you’re familiar with and that’s intermittent fasting. If you could engage in a discipline where you’re restricting your calories to a six- to eight- hour window and having essentially 18 hours of fasting – not having any food or drinking anything except water – then you’re really up regulating the enzymes that are designed to burn fat as a fuel and lowering the glucose enzymes. There’s a decrease in those gluconeogenic pathways.
TS: Yes.
DM: So, I’m wondering if you’ve found and played with intermitting fasting as a useful modality to help people make this transition to the ketogenic diet.
TS: Yeah, well, that’s basically the way it started in the clinic for children with epilepsy. Basically, the child is given a 24-hour and sometimes 48-hour fast – water only. And then the ketogenic diet is introduced in relatively measured and small amounts. The body transitions naturally that way. Intermittent fasting is actually a very strong component of the approach. As you probably well know, there is a blowback against any kind of a fasting or calorie restriction for cancer patients, which is largely unfounded when you look at the physiology. But you’re absolutely correct. It would be good to fast- A three-day fasting, as my students and I have said, is uncomfortable, but it’s certainly doable. It gets your body into a new metabolic state, and then you can apply these therapies. The hardest part about this intermittent fasting – and I look at intermittent fasting as a little bit more than 18 hours. I look at it like 24- to 48- to 36-hour fasting.
DM: How do you implement that regimen? Is just at the initial stage, or is it something you do on a regular basis?
TS: Well, I don’t think most people need to fast all the time unless there’s a real medical need for this. The hardest part, of this fasting is the first three to four days, depending on the individual and how many times they’ve done this. That’s basically trying to break your addiction to glucose. The removal of glucose from the brain elicits the same kind of problems or events as you would if you were addicted to drugs, alcohol, or something like this. You get malaise. You get headaches. You get nausea. You get lightheadedness. You get all the kinds of physiological effects that you would get from withdrawal of any addicting substance. I look at glucose as an addictive substance. It’s an addictive metabolite. Our brains are comforted by having glucose; our bodies are comforted. And when you break that glucose addiction, you have these particular feelings. However, our bodies quickly recognize that this is only transient, and that this guy is not going to bring us back any food. We’re going to now have to upregulate our physiological, evolutionarily conserved adaptive processes. And then the body comes into a new state, and that’s the state that will put the maximum pressure on cancer cells. Even a seven-day water-only fast is still considered intermittent fasting. It’s not what we call long-term therapeutic fasting, which is like 15 to 25 days and longer. The healthy young body can fast for up to 40 days, I’ve seen the evidence for this – without pathology. Longer than that, you enter this pathological state of starvation. Starvation, most people will say, “Oh, if I don’t eat food for two days, I’m starving.” Well, believe me, they’re not starving. Starving is a pathological state where proteins, essential body proteins, are now digested for the fuel of the body. This is a very unhealthy state. You can look at blood metabolites and know that your amino acid levels are shifting toward the starvation state. This is completely not recommended for anyone to do this. Therapeutic fasting is a way (the shorter term) to make the body very healthy over a short period of time. And then the body can transition back to a more normal or a diet with what is considered an organically based health diet. But it certainly has very remarkable health benefits to the body: strengthening the mitochondria network system within the cells of our body. As long as the mitochondria of our cells remain healthy and functional, it’s very unlikely that cancer can develop under these particular states. This is a whole field of physiology that is remarkably healthy but very difficult to do for the majority of people.
DM: Actually, I advocate intermittent fasting as sort of a lifelong approach and really being proactive to set the stage to hit cancers and other chronic degenerative diseases at an early stage. Because I believe that this approach is also hope for many other diseases other than cancer, including heart disease and all these other diseases like Alzheimer’s dementia.
TS: Yes.
DM: One of the things I’ve noticed is it’s really nothing short of a miracle that after you’ve made the transition in primarily making fat as your primary fuel, the desire for these carbohydrates and these sugars just disappears. That’s why I believe most people are afraid of and reluctant to engage in this type of process because they know what it takes to deprive them. They don’t like that feeling when the hunger kicks in. I believe primarily because they’re not adapted to burning the fat. hunger seems to be persistent until they make the transition. To me, one of the major benefits is to be more peaceful and more calm. You do not have to go out and start consuming food that you know is not healthy for you because that’s the only option available. You can go for longer periods until you have access to healthy food.
TS: Right. I agree with you completely. I put that in a chapter in my book on prevention, And what you’re saying is exactly what I’ve said in the book. As long as you can keep the mitochondria healthy – which is when you’re burning fat and ketones – oxygen free radicals within the mitochondria go down. Also, there is a stimulation of autophagy within the cells. The dysfunctional mitochondria are consumed within the cell. And the biomolecules within those dysfunctional mitochondria are then distributed to the healthy mitochondria and the healthy components of the cell. So, we’re actually eliminating the dysfunctional mitochondria and replacing them with a highly efficient energy system within the cell. This happens on therapeutic intermittent fasting. When you’re doing this, you’re actually not only targets and kills cancer cells. I mean, this makes neurons in the brain healthy to reduce the risk of Alzheimer’s disease and cardiovascular disease. This is all linked to the same basic general phenomenon that too much glucose in the bloodstream is not healthy. It leads to inflammation. It leads to cardiovascular disease, which is linked to triglyceride accumulation. It’s linked to a lot of things. It’s also linked to dysfunctional mitochondria, which is the origin of cancer. As long as we can prevent that, individuals should stay a lot healthier. But again, How is it possible for people to set up a regimen of intermittent fasting during the course of their lifetime to reduce these chronic diseases? We think about this and we say, you know… Many religions are associated with intermittent fasting. The problem is it’s not taken to the level of the physiology. In the Christian faith, we have Lent, which is a period of 40 days. If they can fast for Lent for 40 days. That would be very, very helpful. Each religion has a period of fasting.
DM: I’m glad we’re in agreement. The devil’s in the details. I’m wondering if I can tease some of those details out of you with respect to how this is applied. First of all, before we go into that, though, I’m wondering from a physiological perspective. You mentioned the glucose. We know and we believe it to be the primary source of the fuel. But is the beneficial effect more related to the glucose control, which is really the strategy that most endocrinologists and physicians use to control diabetes (and I think is incorrect), or is it an indirect assessment? Because if the glucose is low, then your hormonal shifts occur, and then you lower your insulin and leptin levels, which may have more profound implications. Lowering the glucose serves as an indirect marker for it and is easier to measure than optimizing insulin and leptin levels. TS: You know, I think they’re both purposely connected. The insulin goes up when the glucose in the blood is high, and the insulin goes down when the glucose in the blood is low. This is the normal seesaw effect of this whole process. So clearly, when glucose enters insulin response, when there’s no glucose, insulin is low. Glucagon and other hormones are balanced. Let’s put it this way. Disease basically involves the problem of imbalance. The hormones are not responding to the metabolites the way they should. This is due to excessive disturbances in these balances. So, keeping this whole system balanced by monitoring glucose. Because we know in diabetes – type 1 diabetes – we’ll see tremendous imbalances due to the lack of insulin. that’s a terrible state if you have both high glucose and high ketones together in the bloodstream. These are terribly problematic imbalances. In type 2 diabetes, we have high insulin levels together with high glutamine. The body has become insulin-insensitive. So, again, we have these terrible imbalances,
DM: The traditional physicians today, their average conventional approach for diabetes – type 2 diabetes – is to administer insulin because they believe the bulk of the pathology is related to elevated glucose, not to insulin or leptin resistance. I think it’s a flawed approach. Because by giving the patient more insulin, you actually exacerbate the insulin and leptin resistance; you don’t improve it.
TS: Yeah, that’s correct.
DM: And you’re not addressing the foundational physiological disruption that’s occurring and the imbalances occurring.
TS: You’re right about that. I agree with you. … People can change their diet and lifestyle and manage these diseases. The problem is that it’s hard to do, because of the power of the glucose molecule on the neural functions in our brain .
DM: Well, let’s get into the details. Have you found in your research that there’s a significant difference between sugar as a carbohydrate – pure, simple sugar like glucose, dextrose, or high-fructose corn syrup – and the sugar form of carbohydrates like grains? Have you found that to be a significant difference?
TS: . We’ve shown strong correlations between the pathology of the tumor and the direct relationship to the levels of blood glucose. We’ve done a number of logistic regressions and statistical tests trying to link biomarkers to the kinds of changes we see in the pathology of the tissue, like the vascularization, the angiogenesis, and the inflammation by the NF-kappaB signaling. We’re looking at these molecular signaling pathways in relationship to blood biomarkers. That gives us an indication of how an individual might respond. And then, of course, we don’t just have glucose. There’s the glutamine issue, which is a very interesting aspect of this and which is far from being resolved. I think most oncologists who do cancer metabolism recognize that glucose is the prime fuel for driving the tumor. However, we and many others have shown that glutamine and glucose together act powerfully and synergistically on the growth of that tumor cell. These two fuels work together in concert to provide a continual growth of that. Glucose is certainly important. It’s very targetable and effective. But we need to recognize that there are other fuels that will drive this tumor as well.
DM: Well, thank you for bringing that up. I was going to discuss that. Now, glutamine, for those who are watching this that are not familiar with this, it’s a simple amino acid, one of the most common ones. It’s an amino acid that’s part of protein. It sounds like this dual strategy of lowering the glucose and glutamine are also useful. How is that practically implemented? Do you just recommend a certain amount of grams of protein per kilogram of body weight or lean body mass? Or is there just a simple restriction of glutamine?
TS: Well, you’re right about that. We haven’t worked on those food sources or diets that might facilitate lowering glutamine. Glutamine is the most abundant amino acid in our bloodstream. It plays a role in a variety of other metabolic processes. Getting glutamine down with drugs can be done. Phenylbutyrate (Buphenyl) is metabolized to phenylacetate, which then binds glutamine, and then glutamine can be excreted. This has been used for certain rare amino acid disorders. That drug has been shown to be effective in certain cancers. we’ve shown is that many of the metastatic cancers are derived from macrophages, either stabilized macrophages or fusion hybrids between macrophages and cancer stem cells. The macrophage is the most powerful cell in our body. It evolved to kill bacteria. It evolved to reconfigure tissue in hypoxia, injury, and things like these. These cells can phagocytize even if we deprive them of glucose and glutamine. These cells could eat another cell in the environment, strip off those fuels, and actually get energy from phagocytizing cells. Here’s another issue that people need to be [aware of] and that those of us who are working in the field are aware of. We can create one of these ketogenic diets and suppress the cancer and have people live a healthier and longer life. But at the same time, we know that some of those cells can actually hang out in the body and potentially survive by the engulfment of materials within our body to give them the glucose and glutamine that would be deprived. It’s not because cancers have some magic power; it’s because they are derived from the other cells . Gluconeogenesis can actually be generating glucose from other sources of proteins
And lowering the glucose. We’re very clear about lowering grains and anything that would raise your blood sugar. But there are, of course, two other macronutrients left: fat and protein. Many of the Paleo people are too concerned about high amounts of protein. And according to your research, that could increase the glutamine – most likely it will – and that could be problematic. Dr. Rosedale has found through his research this pathway, mammalian target of rapamycin (mTOR), which is, of course, an anti-cancer drug. This ancient pathway was discovered about 10 years ago. It’s controlled by lowering your proteins. That could be another metabolic pathway that actually normalizes the cancer growth. I’m wondering if you can comment on that about approach.
TS: Well, we did some studies on this with our model of glioma. We looked at mTOR. It wasn’t changing as much as some of the other pathways, like the Akt signaling pathway (protein kinase B), HIF-1alpha pathways, and the vascular endothelial growth factor (VEGF) metabolites. The mTOR in our model was not that dramatically changed by these metabolic therapies. But I know others have reported it, and this could be an important component for certain other kinds of cancers. But my limited work with this did not demonstrate this to be a major issue, at least in this glioma model that we looked at.
DM: In the glioma setting, it had a low protein intake, too? About one gram perkilogram?
TS: It was a test for some calorie restriction. This is another issue when we talk about intermittent fasting or calorie restriction. Calorie restriction in the mouse is not the same as calorie restriction in humans. Human has seven times lower metabolic rate than the mouse. So, therapeutic fasting in humans actually mimics calorie restriction in the mouse. That’s what you’re talking about when you talk about intermittent fasting. Water-only fasting for several days is comparable to a 40-percent calorie restriction in the mouse. When we did the diets that had different amounts of carbs, proteins, or things like that, it was the amount of food consumed that was related more to the issues. I should say the amount of food consumed that was related to the caloric intake. We linked a lot of this to just simply the caloric intake. We showed that you could give animals a high-fat, low-protein diet, give it as much as they want (add zero carbs in this diet). Their blood glucose was just as high or higher than the mice that were eating the protein-carb diet. It was more or less related to the total consumption of calories. Calories, most of them come down to glucose. Proteins will be metabolized to glucose. Carbs are metabolized to glucose; fats are not. The only part of the fat would be the glycerol backbone of the triglyceride, which is conjugated in the liver to make glucose. Even parts of fats can be made into glucose. The whole issue then becomes, you know… We don’t get any therapeutic benefit either in epilepsy or cancer when we allow the animals or people to eat as much of these high-fat diets as they want. We get no therapeutic benefit. Therapeutic benefit comes from the restriction of the calories in the diet. Now, some people find that these diets are unpalatable. And therefore, while I eat all I want, but what they want is actually much less than what they would normally eat. It’s only because the diet doesn’t taste good. And the mice do the same thing. Some of these ketogenic diets the mice absolutely dislike. And they get this tremendous benefit. You say, “Oh, look at the mouse, say, get this ketogenic diet and get all this.” You notice his body weight is falling and get all this other stuff. You know that’s coming from the reduced caloric intake. But another ketogenic diet, one that’s flavored with artificial sweeteners and these kinds of things, the mice eat large amounts of this stuff. They get all of the health problems – as diabetics, it makes the cancer grow faster, etc. It doesn’t help them at all. Why we use the ketogenic diet or a low-carb, low-protein diet is simply a way to take the sting out of a therapeutic fast. Because people, as long as the glucose and ketones can get into the metabolic range (and you can do it with eating small amounts of a fat diet rather than therapeutic fasting), then that just makes people feel a little bit better about how they’re doing this rather than feeling that I’m starving to death.
DM: That’s interesting. I haven’t heard that before that the actual calorie restriction was an important part of the equation, and that you really can’t go on limiting fat and increasing calories because of what you mentioned about glycerol being converted into glucose. TS: Yeah. DM: So, let’s talk about the specific types of fat. What percentage of fat do you find your patients typically consuming as a percentage of calories? The types of fats that we recommend are healthy fats. Obviously, you don’t want them to do trans fats (I mean, that’s not controversial at all), but also to limit processed vegetable oils like omega-6s and replace them with more of the saturated fats like coconut oil, butter, macadamia nuts (which is also low in protein), and avocados. Have you found that really guiding people on the specifics and the details of the fat and helping them choose healthier ones have made a big difference?
TS: Well, I think that is definitely a very important point. The reason why we use medium-chain triglycerides – coconut oils, butter, macadamia nuts, and these kinds of things – is they have saturated fats. The saturated fats are converted to ketones much, much more readily than polyunsaturated fats. . Very few studies have been done on combining ketogenic diets with, say, omega-6 or omega-3 fatty acids and these kinds of things to see whether or not we can actually significantly improve the kind of metabolic approach that we’re using to manage the disease. We’re at the very beginning of our quest to manage these complex diseases using non-toxic, natural approaches. We need to know how to balance these to get the maximum effects.
DM: What has been your experience when you’re recommending these strategies and the research you’ve done over the past 25 years to clinicians or to oncologists who are treating cancer patients? Is there a reluctance? Are you seeing an opening to the door, where they’re beginning to adopt these approaches and integrate them with their current therapies?
TS: Well, I think there are a couple of issues in relationship to that question. There are a number of physicians who are realizing that this is a way to improve the health of their patients. On the other hand, there are probably a far greater number of individuals, physicians, and healthcare givers who are reluctant to even consider this as an alternative to what their standard practices are. The reason for that reluctance, in my view, is just simply the lack of knowledge of the concepts involved in this. And also the lack of training. I don’t think a lot of medical schools are training physicians to understand the power of nutritional capabilities and how to actually use that as a tool to manage health. This strategy is sort of a shocking approach that will radically reduce your risk for every one of those diseases. I’m wondering if you can summarize your five best tips on how to implement this for a preventive strategy.
TS: Well, . Intermittent fasting is a way to prevent cancer. I mean, I think this is clear not only with cancer, but as you said a lot of other diseases. But you did raise one issue that we need to address, and that is the so-called provocative questions from the National Cancer Institute. One them relates to why do people with Alzheimer’s disease have much lower risk for cancer. “I don’t really know why people with Alzheimer’s disease would have a reduced risk for cancer.” We know that the same preventive strategies would help them as well as the cancer patient. But what is it about them that make them have a lower risk? It turns out that people with Alzheimer’s disease – it’s been documented – are hypometabolic. The glucose transporters on their cells are underrepresented. They can’t get the fuel that would drive cancer. Their blood sugar levels are low, so they’re hypometabolic. The bigger question I think is, what’s making the person with Alzheimer’s disease hypometabolic in the first place? These ketogenic diets and things like these will actually enhance the metabolic deficiency of tissues. In one case, you need to enhance it to prevent Alzheimer’s. And in another time, you need to reduce it to prevent cancer or target the tumor. The whole thing has to be viewed in the context of the disease and the metabolic state of the individual. But I think for cancer prevention, eating the healthy kinds of foods, the balances, maintaining a metabolic homeostasis, one that’s related to health rather than inflammation. Inflammation is a culprit linking many of these diseases together. Body inflammation is linked to higher blood glucose levels, and this puts you at risk for all these other. Any health strategy that reduces inflammation, reduces glucose and these kinds of things, will put you – not no risk, but certainly – at a lower risk for many of these kinds of diseases certainly. DM: The other group of people who I would like to address is the people who actually have cancer or someone close to them have cancer. And that’s a number of people. For those, it would seem – from my perspective and what I’ve learned over the years – that it’s nothing short of reprehensible medical malpractice and negligence not to integrate this type of strategy into their cancer treatment plan. Anyone can do their due diligence to figure out whatever treatment approach you’re going to have. This can easily be integrated into whatever plan you’re going to do. I just think it’s absolutely crucial. There’s no cancer that doesn’t benefit from treating this from my perspective or from my understanding. Perhaps you can elaborate on that. Why don’t you address the strategies for them? I think that, my guess is that the number one approach is to get your book Cancer as a Metabolic Disease and start following the recommendations in there and find an enlightened healthcare clinician who can cooperate with you, because you need someone who can act as a coach and help you through that. If you can expand and elaborate on that, that would be great. TS: Well, this is a very important point. At first, my personal view is that we can manage this disease. We can prevent the disease for sure. We can manage the disease in those individuals that have it through the metabolic approach. The issue that I have come to recognize is, you know, radiation, oncology, chemotherapy, and all these kinds of stuff. I agree with you: some of these are horrific treatments that can do more damage than good in the long run. They’re quick fixes to a much more complicated problem. However, I think that dovetailing radiation with metabolic therapy could significantly improve the therapeutic benefits of radiation. Although I’m very much opposed to radiation, I think that when applied together with a metabolic therapy, the outcome would be better. The patient will have less collateral damage, and the therapy could actually have much more significant positive benefit. Clearly, there will be a hybridization. There has to be a hybridization of these approaches for the benefit of the patient. Only in time will it become recognized that we don’t need the toxic approach. Let me give you an example. We have a paper that will be coming out very shortly (my colleagues and I there at the University of South Florida and here at Boston College), showing how dovetailing the ketogenic diet or the mildly restricted diet with hyperbaric oxygen therapy shows powerful synergy. Both radiation therapy and hyperbaric oxygen therapy will create increased oxygen free radicals, which is designed for killing the cancer cells. One does it in a non-toxic way, and the other does it in a very toxic way. Now, of course, radiation won’t work in a hypoxic environment where oxygen can’t get in there to facilitate the killing of the cancer cells with radiation. This is one of the failures why radiation doesn’t work for a lot of the cancers. However, we can bring the oxygen into the tissues and radiation will work a lot better, but so will hyperbaric oxygen therapy, which is much less toxic. We know that we can dovetail these approaches together. We know that some of the blockbuster drugs like Gleevec and Herceptin, they target the same signaling pathways that does the ketogenic diet. So, why would we take these horrific drugs that do have good benefit but also have adverse side effects? But can we get the benefits without the adverse side effects? Or will the drugs work even better at lower doses together with the diet? Again, this is a hybridization of approaches that’s only going to benefit the patient in the long run, make their life a lot easier to live, and reduce the side effects from these other kinds of therapies. And then eventually, like anything that happens, there will be in the future more reason to transition over to the metabolic therapy as the prime approach to the management of the disease. Healthcare providers will eventually come to know this. It’s just that at this point in time, this is largely unknown. DM: All right, one last question, and that is on your personal observations and studies. I think it’s probably fair to summarize that the current approach, traditional approach – or conventional approach would be a better term for it – for most cancers is simply cut, poison, and burn. Cut is surgically cutting the bulk, which is certainly appropriate. There’s no question. I mean, it can save a person’s life. TS: Yeah. DM: Burn would be radiation. And that may be appropriate, too. It’s certainly more dangerous. But the poison or the use of chemotherapy, in my view – I would be interested in your perspective – is rarely ever indicated. I mean, that is the very reason why it works: it destroys your immune system. As you mentioned or alluded to earlier, we need our immune system to cancer. You’re destroying the very tool your body has to defeat this thing. Even though it may be short-term successful, I think long-term ramifications are just disastrous and, in many cases, will kill the patient. I’m wondering if you can comment on that. TS: Well, certainly, in my book I have a section on it. I reviewed some of the latest information on some of the so-called targeted therapies and things like these. The problem here is that they’re looking at major advanced cancer being manageable and [inaudible 1:08:59] as three, four, to six months longer survival. This is…. DM: True. TS: This is not the real… This is fantasy land. I don’t know what to say. We think the cure is six months longer life. I don’t think this is… It’s been shown that these types of drugs that are very, very costly with rather significant adverse effects are not the long-term solution. But what’s driving this, though, is the genome projects. This again comes back to the heart of the problem. As long as you think that this is a genetic disease, you’re going to persist with these kinds of toxic therapies that have little long-term benefit for the patient. This again comes to the core of the problem, which is: is this a metabolic disease, or is it a genetic disease? I think the shift away from these kinds of targeted chemotherapy will likely diminish once people realize this is not the core problem. [—– 1:10:00 —–] Again, this is going to require an evolution of thought and approaches. It will take some time, and these huge financial investments on the part of the academic community and on the part of the pharmaceutical community. The academic and pharmaceutical communities are closely linked in how to deal with cancer. But this is often divorced from the real-world situation that the patient is dealing with. We have these large institutional viewpoints on how to manage this disease that are largely ineffective, when the solution to the problem is if you change your view on what the nature of the disease is, you’re going to see much greater benefit for the patients. DM: Well, I want to thank you for all your pioneering work in helping us and the medical community come to a deeper and better appreciation of this foundational concept and everything you’ve put together so far. So, if someone’s interested in more specifics, they can certainly get your book, Cancer as a Metabolic Disease, on Amazon. Do you have a website or any other resource that you could recommend? TS: Well, we have a Facebook page, I think, on the book, that was set up. My website is at the Boston College Biology Department. We keep up-to-date on that. But primarily, those are the major sites on this. I get so many emails from cancer patients all over the world. As I said, not being a physician, I cannot recommend any kind of a… So, what I do is I basically get them in contact with those physicians (who can help them and understand the situation) and nutritionists that help these people. But we certainly need more effort into this area. DM: That’s a very good point. Perhaps we can close on that. Is there any list of physicians who know how your approach is and are able to help, guide, and approach people in implementing this? Is there some compiled list, one that you can recommend? TS: Well, right now there’s not a compiled list. But there are certain physician groups – and I’ll be speaking to some of these groups – with the hope of explaining better how they can use their talents and experience to help implement and better understand the concepts that we’re doing. This then will blossom into a much more concerted effort to do this. But I think physicians are basically interested in the well-being of their patients. If they can do something to help them and they can see an immediate benefit from this, they’re going to be onboard with this whole concept. How can this be dovetailed or integrated into our healthcare policies is another issue. Will insurance companies begin to cover some of the costs? One would think the insurance companies would be onboard with this. I mean, you can potentially heal patients at a much lower cost. It’s just that they would be the ones that I would think would have the most interest in certainly this kind of approach. DM: Absolutely. I think that’s one of the reasons. I’m convinced this is one of the reasons why this approach persists. It’s because it’s such a lucrative field. I mean, these kinds of drugs can be tens of thousands of dollars a month or more. TS: Right. DM: Frequently more. The cost of the many metabolic therapies you’ve outlined is, I don’t know, a fraction, probably less than one-tenth of one percent of the cost of these drugs. It’s essentially not… I mean, the cost for a person to implement this, there’s really no cost. It’s just your committed willingness and dedication to applying these principles. Actually, it seems to reduce the cost of your food, because you’re eating less food. TS: Yeah, that does it. I mean, it seems like a no-brainer. We have a healthcare crisis. The cost of healthcare in this country continues to rise. This is the end of the financial well-being of the nation. I mean, we have a major approach that can certainly reduce healthcare costs significantly. It’s unbelievable that we would pursue costly drugs that are potentially toxic when you can get the same benefit from a little education and a little perseverance and discipline. DM: It’s not so unbelievable when you look at the numbers involved. There’s a very good reason why that persists, and it’s in the process of bankrupting our country. But I believe that’s a good thing. I tend to approach life from the inverse, that these events that occur seem to be a disaster, such as our sort of looming healthcare crisis is and the destruction of our economy due to the inflation. Well then, people become desperate because resources become limited. Then you are forced. You have no choice. You can’t afford these drugs. You can’t afford surgeries. You have to rely on simple strategies to work. There can hardly be any simpler and less expensive strategies than yours when it comes to the treatment of cancer. I think we’re going to be forced eventually because the system’s going to… TS: Well, the other issue, too, of course, is that many cancer patients fear the treatments as much or more than they fear the disease itself. Many people [inaudible 1:15:26-27] complication of cancer. What does that mean? That means the treatments lead to death rather than the disease. So, why would one want to do that rather than taking the alternative that you can benefit from without putting your health through that situation? DM: I couldn’t agree more. My girlfriend just had two relatives in the last few weeks who were diagnosed for cancer. And as a result of that, I said, “Well, let’s give them a healthy diet and treatments.” TS: Yeah. DM: It’s not, unfortunately, an uncommon experience. TS: I know. DM: It’s truly [inaudible 1:16:09]. TS: No, it’s a tragedy. DM: [inaudible 1:16:11] All right, again, I want to thank you for everything that you’ve done, for everything that you will continue to do, and your pioneering, innovative natural approach that really addresses this foundational cause of diseases. I’m looking forward to see the fruits of your efforts in the future. TS: Thank you very much. We’re all hoping for that.
